Various factors can modify coastal phytobenthic populations. In recent decades, such changes have been correlated with various human activities along the coast, the introduction of exotic species and global climate change. Increasing human impact on coasts includes residential settlements, ports, breakwaters, docks and discharge of urban and industrial waste water. Pleasure boating and mooring in areas populated by marine phanerogams is another disturbing factor. All this has undoubtedly reduced the area of meadowsof the seagrass Posidonia oceanica, which are now believed to be decreasing significantly throughout the Mediterranean Sea. Finally, warming of the Mediterranean by the greenhouse effect (Bradley, 2000) may already have caused changes in phytobenthic populations and favoured allochthonous species that have largely been introduced and spread by shipping (Boudouresque and Verlaque, 2002).
In recent years, the number of introduced macroalgal species has risen in all marine ecosystems with increasing marine traffic (through fouling and deballasting of water), aquaculture and commercial activities (Boudouresque and Ribera, 1994; Verlaque, 2001; Sfriso and Curiel, 2007). In the Mediterranean Sea, 85 introduced macroalgae have been listed and eight of them are considered invasive (Boudouresque and Verlaque, 2002a). The invasive Caulerpa racemosa var. cylindracea (Sonder) Verlaque, Huisman, Boudouresque (Bryopsidales, Chlorophyta) (hereafter: C. racemosa), introduced in the early 1990s from the south-western coast of Australia (Verlaque et al., 2003), has spread swiftly along Mediterranean coasts in the last 15 years (Ruitton et al., 2005a; Piazzi et al., 2005). The ecological role of C. racemosa is still debated, however most authors consider it harmful to autochthonous phytobenthic communities, especially to algae constituting turfs and to a lesser extent to taller species (Boudouresque and Verlaque, 2002b; Piazzi and Balata, 2007). There is some consensus that dense cover of a large number of established indigenous species can be a major factor in reducing the probability of successful invasion (Ceccherelli et al., 2000). Observing the spread of C. racemosa in situations of environmental crisis and rarefaction of autochthonous populations, authors of early studies claimed that it was a stress-tolerant species and a possible indicator of active environmental disturbance (Buia et al., 1998).
The coastal stretch at Ansedonia (Orbetello, southern Tuscany, Italy; Figure 1) had a back reef area of Posidonia oceanica (L.) Delile with a habitat characterized by distinctive mixed meadow communities of macroalgae and seagrass. Since 2003 the mixed meadow suddenly disappeared (Lenzi et al., 2007), replaced in 2004-2005 by C. racemosa, which spread shoreward from the barrier reef of P. oceanica. C. racemosa biomass showed an increase of two orders of magnitude between July 2005 and July 2006 (Birardi et al., 2008), confirming the considerable substrate-covering capacity and rapid development shown by this species in other parts of the Mediterranean Sea and also its aggressiveness on shallow sheltered bottoms with dead mattes.
.png)
Figure 1 The study area at Santa Liberata (southern Tuscany, Italy)
|
As a result of this sequence of events, we considered it important to continue monitoring the phytobenthic settlement dynamics of Santa Liberata back-reef and barrier-reef areas. We therefore monitored cover and biomass of phytobenthic flora in the back-reef in order to assess any differences with respect to the previous spread of C. racemosa and to describe the biomass ratio of this species to other phytobenthic species. Our aims were: to understand the role of this invader in the colonisation of degraded areas; to assess whether its aggressive nature can effectively prevent reconstitution of the original community; to assess whether it can also attack and create critical conditions for P. oceanica reef areas.
1 Results
Floristic lists with specific cover values for the survey of August 2011 are reported in Table 1, where they are compared with July 2005 and 2006 survey results. The number of species changed from 32 to 30 and 38 in 2005, 2006 and 2011, respectively. The species with highest cover were: Jania rubens v. rubens, Padina pavonica, C. racemosa, Cladophora prolifera, Penicillus capitatus and Nanozostera noltii in all three years, and also Dictyota dichotoma and Cladophora sp. in 2011. C. racemosa showed a decrease in cover in 2011, returning to 2005 levels, while N. noltii increased its cover in 2011. Phytobenthos still consisted mainly of a thin compact mat, dominated by the dense texture of J. rubens and Cladophora spp., with a prevalence of the latter, from which emerged sparse N. noltii leaves and tufts of P. pavonica and D. dichotoma, that sometimes developed into extensive patches.

Table 1 List of macrophytes and their cover in the two plots (a, b) of the back-reef area of the Posidonia oceanica barrier reefs off Santa Liberata (Orbetello, Tuscany, Italy), in July 2005-2006 and August 2011
|
The results of correspondence analysis of cover data of the lists of species in 2005, 2006 and 2011 are reported in the biplot of Figure 2, where species are given progressive numerical values in the alphabetical order of Table 1. We found that: a) the species coded 6, 8, 14, 15, 18, 20, 21, 22, 40, 43, 44, 46 and 39 (superimposed on the graph of Figure 2) were abundant in 2011 and absent in the other two years; b) species 34, 25, 11 and 9 were present in 2005 and 2006 and absent in 2011; c) species 19 was only present in 2005; d) species 17 was present in 2006 and 2011; e) species 1, 2, 5, 10, 12, 16, 24, 26, 28, 30, 31, 32, 33, 36, 37, 38, 41, 42, 45 and 47 (superimposed on the graph of Figure 2) had no influence on the results because they were always present. The year 2011 plotted on the positive semiaxis of the abscissa because it was characterised by abundance of the species listed in point a), confirming the peculiar nature of 2011 compared to the other two years. The years 2005 and 2006 plotted on the negative semiaxis, being characterised by the species described in point b), confirming their similarity in terms of presence of certain species, and dissimilarity with respect to 2011.
.png)
Figure 2 Result of correspondence analysis of the data
|
According to Boudouresque (1984), the species that developed in the back-reef (LB in Figure 1) in the three years (Table 1) mostly belong to the following ecological groups: Photophilous–Infralittoral-Thermophilous (PhIT), Photophilous–Infralittoral-soft bottom (PhISt), Photophilous–Infralittoral–Quiet environment (PhIQ), Photophilous–Infralittoral–Harbours (PhIH), Antisciaphilous (AS), Sciaphilous–Infralittoral–relatively Quiet environment (SIQ), Sciaphilous–relatively Quiet environment (SQ) and Sciaphilous-Infralittoral (SI). The conditions in the study area were therefore relatively calm and subject to considerable summer warming, in some respects similar to a harbour environment. The species observed for the first time in 2011 also had these characteristics. The ratio of the number of photophilous species to sciaphilous species (Ph:Sc) was 1.6, 1.9 and 1.3 in 2005, 2005 and 2011, respectively.
Macroalgal biomass is reported in Table 2. Total biomass showed a large increase between July 2005 and July 2006, while in August 2011 it dropped sharply (about -65%). C. racemosa showed the same trend, its biomass increasing by two orders of magnitude (+8173%) between 2005 and 2006 and decreasing by 96% between 2006 and 2011, the worst performance of all macroalgae. These variations of C. racemosa were clearly reflected by C.r.:T ratios (Table 2).
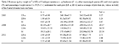
Table 2 Biomass (g dry weight m-2 ± SD) of Caulerpa racemosa v. cylindracea (C. r.), other macroalgal species (O) and total species (T), and percentage weight ratios C.r.:T (% C.r.) estimated for each plot (LB-a, LB-b) and as average of plot data (m, values in bold) of the Santa Liberata back-reef area |
During theobservationsconducted inthe whole back-reef area, C. racemosa proved widespread butwithfew smallthalli,inline withthe results shown by the plots. The species was not observed in theemerging stretch of theP. oceanica meadow that forms the barrier-reef.
2 Discussion
When Lenzi (1987) described the P. oceanica barrier reef at Santa Liberata, besides the seagrasses C. nodosa and N. noltii, he reported the macroalgae Caulerpa prolifera, Cystoseira barbata J. Ag., Halimeda tuna Lamour, Sphaerococcus coronopifolius (Good.-Woodw.) C. Ag., Rytiphloea tinctoria (Clem.) C. Ag. and Alsidium corallinum C. Ag. This mixed meadow collapsed, starting in 2003, and by 2005 not a trace of it remained.
In the observations made between July and October 2005 and 2006 (Lenzi et al., 2007) and in those of the present study (August 2011), none of the species listed by Lenzi (1987), except N. noltii, was found. Since 2005, phytobenthic populations have been unstructured and dead Posidonia-mattes have become covered with thin algal mats consisting mainly of J. rubens and Cladophora prolifera. Another two species, never previously observed along the southern Tuscan coast, were found instead: Caulerpa racemosa and Penicillus capitatus Lamarck (Bryopsidales, Chlorophyta). Both species were observed on the dead mattes of P. oceanica left by the mixed meadows, while P. capitatus also colonized the sandy bottom, distributed in isolated patches of a few square metres at most. C. racemosa was widespread, its stolons entwined in the thin algal mats. The explosion of this species between 2005 and 2006 in the shallow water of LB area (Figure 1), is in line with this alga’s high phototolerance (Raniello et al., 2006) and with the abundance of organic detritus in this relatively quiet area, condition favorable to growth according to Piazzi et al (2007). Perhaps these two species had already been present for some time, because it seems unlikely that they came just a year after the perturbing event. They were probably already present in microhabitats, escaping previous, superficial observations.
Successiveobservations (Lenzi, not published) showed rapid changes in facies, e.g. between June and September 2008, there was a bloom of Cladophora sp. that produced free-floating balls of pleustophytic thalli. This species grows in height and forms pedunculated tufts from which aegagropilous bodies detach. Cladophora balls formed beds in the deeper areas of LB, where waves and currents carried them into the open sea. While this still was an important development, there were no further high blooms of Cladophora in the following years.
Despite a significantoveralldecrease inbiomass, the phytobenthic community observed in 2011 seemed better structured. This is also sustained by values of the Ph:Sc ratio, which was highest in 2006, during maximum "degradation”, and lowest in 2011, and by the 27% increase in the number of algal species present in the mat, with respect to 2005-2006. All the species were still typical of PhIQ, PhIT, PhIH, SIQ, SQ and SI.
A decrease in macroalgal biomass of about 65% between 2006 and 2011 was mainly due to the loss of relatively large macroalgae, such as P. pavonica, and probably to dominance of Cladophora spp. with respect to J. rubens in the mats (Table 1). The greatest reduction in biomass was that of C. racemosa. The drastic nature of the decrease in this species in 2011 was not evident from its cover values (Table 1) because a small quantity of thalli covered most of the bottom where it had spread in 2006. This species seems to have stopped developing, and while it remained widespread, underwent a drastic 95.7% decrease in weight.
Fromthis seriesofevents, including the fact that C. racemosa has not yet penetrated the meadows of Posidonia oceanica, we propose the following explanation of the observations: 1) as a result of disturbance, such as the excessive heating that occurred in 2003 and/or increasing impact of bathing from 2002 (Lenzi et al., 2007) to 2008, the plant communities in LB underwent profound deterioration; 2) in the phase of impoverishment and destabilization of the phytocoenoses, between 2005 and 2006, C. racemosa showed massive development; 3) there followed a phase of variability of phytobenthic settlement, characterized by the development of C. racemosa and Cladophora balls; 4) the recent period seems to involve reorganization of plant community structure, probably in the direction of restoration of the typical phytobenthic community, with a sharp decrease in the “invasive” C. racemosa, stability of P. capitatus, a thermophilous species, and recovery of the seagrass N. noltii.
Our hypothesis is that “invasive” species become invasive when the plant community is damaged. When the causes of damage cease, the typical components of the phytocoenosis tend to recover. Reconstitution of the community may occur by a specific succession if settlement and growth of autochthonous species are able to occur before the sea and other forces destroy edaphic characteristics.
Few studies have focused on self-restoration of biocoenoses after destructive events, and none after an invasion by C. racemosa. One experiment on recovery of the macroalgal community after manual removal of C. racemosa proved completely ineffective (Piazzi and Ceccherelli, 2006), probably because the causes of impoverishment of the original community persisted. Recently, Tsiamisis et al (2013) documented the reconstitution of benthic macroalgal communities and the return of oligotrophic conditions after cessation of a source of eutrophication, an urban sewage outlet. Reconstitution of original biocoenoses is an expression of ecosystem resilience. We sustain that resilience can also be expressed towards aggressive invasive species, provided strong perturbation (human impacts or climatic variations) does not persist. The aggressiveness of a species is clearly linked to environmental conditions and species rightly described as “invasive” develop well in a wide range of conditions. However, there may also be blooms of species not considered invasive. For example, Alsidium corallinum C. Ag., a typical marine species, developed in an unexpected way in Orbetello lagoon in 2007. Initially barely detectable, it reached a biomass of thousands of tons in a single year. This was due to transient conditions favourable for its growth (Lenzi et al., 2012). Naturally, such cases are more likely when an ecosystem is not in equilibrium and does not express stable community structure. In other words, use of the term “invasive” for an allochthonous species, such as C. racemosa v. cylindracea, should be reconsidered. Invasiveness should rather be regarded as a potential attribute, probably common to many other species. This is sustained by the fact that more than 10 years ago, Ceccherelli et al (2000) found that fast spread of C. racemosa in a Posidonia meadow was related to the availability of free substrate and that the species could not penetrate a dense meadow. More recently, Casu et al (2009) demonstrated an important trophic role of the species for zoobenthic organisms on rocky substrates. Thus the invasive potential and danger of C. racemosa v. cylindracea is expressed when environmental conditions permit, as in the case of biocoenoses impoverished for different reasons.
3 Materials and Methods
The study area lies off Santa Liberata beach, on the right side of the outlet channel connecting Orbetello Lagoon to the sea (Figure 1). Close to the artificial channel, an emerging P. oceanica barrier-reef defines a back-reef area up to 2.5 m deep and about 5000 m2 in area (LB, Figure 1). In the past, this area hosted a mixed meadow of the seagrasses Cymodocea nodosa (Ucria) Ascherson, Nanozostera noltii (Horneman) Tomlinson et Posluzny, and the chlorophycea Caulerpa prolifera (Forsskål) J.V. Lamouroux (Lenzi, 1987). Since 2003, increasing rarefaction and regression of the mixed meadow has been observed in LB by aerial photography. Field observations conducted between July and October 2005 and 2006 (Lenzi et al., 2007) showed rapid progressive invasion by Caulerpa racemosa.
The area was again viewed by scuba early in August 2011, in order to determine the current vegetation assemblages. The two 5×5 m2 sampling stations (1.5 m deep) established in the back-reef area during the 2005-2006 research, were used again in the field research of 2011 (LB-a and LB-b; Figure 1).
Samples were taken from these plots to determine species and plant biomass and a survey was carried out to determine specific cover. In the survey, 25 photographs of the phytobenthos were taken in each plot in a 20×20 cm frame, positioning the frame according to a fixed scheme. For each picture taken, a sample of macroalgae was collected for species determination.In each image, species cover (Ri) was determined by a phytosociological method (Boudouresque, 1971) with the aid of a grid. The average cover of each species (RMi) was calculated for the plots. Cover scores 1, 2, 3 and 4 for <5%, 5%~25%, 25%~50% and >50% cover, respectively, were assigned to phytobenthos species. The result was compared with those of late July 2005 and 2006 (Lenzi et al., 2007), carried out by the same method. For comparison of the lists of species presence and cover for 2005, 2006 and 2011, we used explorative correspondence analysis with the software package ade4(Chessel et al., 2004; Dray and Dufour, 2007; Dray et al., 2007).
Fifteen samples were collected from a 20×20 cm square in each plot (6000 cm2 total) for determination of biomass. There were many rocks in LB-a, from which we scraped the covering of vegetation. Samples were washed to remove impurities and divided into two portions, isolating Caulerpa racemosa (C.r.) thalli from the other macroalgal species (O). The two macroalgal portions were then oven-dried at 85℃ for 24 hours. Total biomass (T=C.r.+O) and the percentage of C. racemosa on total biomass (%C.r.=C.r. * 100 * T-1) were then computed.
Only a simple survey was carried out in the P. oceanica barrier-reef meadow and other parts of LB, mainly to establish the degree of invasion by C. racemosa.
Authors' contributions
ML conceived the experimental design, participated in the field survey, sampling and species determinations and drafted the manuscript. FB participated in the field survey, sampling and species determinations. MGF performed the statistical analysis and helped draft the manuscript.
Birardi F., Lenzi M., Franchi E., Solari D., Roffilli R., Gennaro P. and Focardi S., 2008, Spread of Caulerpa racemosa var. cylindracea in Back-reef Areas (Tuscany southern coast), Biologia Marina Mediterrranea, 15(1): 256-257
Boudouresque C.F., 1971, Méthodes d’Etude Qualitative et Quantitative du Benthos (en particulier du Phytobenthos), Tethys, 3: 79-104
Boudouresque C.F., 1984, Groupes Ecologiques d’Algues Marines et Phytocenoses Benthiques en Mediterranée Nord-Occidentale : une Revue, Giornale Botanico Italiano, 118 (2): 7-42
Boudouresque C.F. and Ribera M.A., 1994, Les Introductions d’Espèces Végétales et Animales en Milieu Marin – Conséquences écologiques et économiques et problèmes législatifs. In : Boudouresque, C.F., Meinesz, A., Gravez, V. (Eds.), First international workshop in Caulerpa taxifolia. GIS Posidonie Publications, Marseille, pp. 29-101
Boudouresque C.F. and Verlaque M., 2002, Assessing Scale and Impact of Ship-transported Alien Macrophytes in the Mediterranean Sea. In: CIESM (ed) Alien Marine Organisms Introduced by Ships in the Mediterranea and Black Seas. CIESM Workshop Monographs 20: 53-61. Monaco.
Boudouresque C.F. and Verlaque M., 2002a, Biological Pollution in the Mediterranean Sea: Invasive Versus Introduced Macrophytes, Marine Pollution Bulletin, 44: 32-38
Bradley R., 2000, 1000 years of climate change, Science, 288: 1353-1354
Buia M.C., Petrocelli A., Saracino O.D., 1998, Caulerpa racemosa Spread in the Mediterranean Sea: First Record in the Gulf of Taranto, Biologia Marina Mediterranea, 5(1): 527–529
Casu D., Ceccherelli G., Sechi N., Rumolo P. and Sarà G., 2009, Caulerpa racemosa var. cylindracea as a Potential Source of Organic Matter for Benthic Consumers: Evidences from a Stable Isotope Analysis, Aquatic Ecology, 43: 1023-1029
Ceccherelli G., Piazzi L. and Cinelli F., 2000. Response of the Non-indigenousCaulerpa racemosa (Forsskål) J. Agardh to the Native Seagrass Posidonia oceanica (L.) Delile: Effect of Density of Shoots and Orientation of Edge of Meadows, Journal of experimental Marine Biology and Ecology, 243: 227-240
Chessel D., Dufour A.B. and Thioulouse J., 2004, The ade4 Package-I- One-table Methods, R News, 4: 5-10
Dray S. and Dufour A.B., 2007, The ade4 Package: Implementing the Duality Diagram for Ecologists, Journal of Statistical Software, 22(4): 1-20
Dray S., Dufour A.B. and Chessel D., 2007, The ade4 Package-II: Two-table and K-table Methods, R News, 7(2): 47-52
Lenzi M., 1987, Le Récif-barriére de Posidonia oceanica (L.) Delile de Santa Liberata (Toscane, Italie): Cartographie et Biometrie, Giornale Botanico Italiano, 121 (3-4): 155-164
http://dx.doi.org/10.1080/11263508709429373
Lenzi M., Franchi E., Giovani A., Micarelli P., Perra G., Roffilli R., Solari D. and Focardi S., 2007, Change in the Phytobenthos Settlement Along the Santa Liberata Coast (Southern Tuscany, Italy), Proceeding of the Third Mediterranean Simposium on Marine Vegetation 27-29/3/07 Marseilles, UNEP, MAP, RAC/SPA, 88-95
Lenzi M., Gennaro P., Renzi M., Persia E. and Porrello S., 2012, Spread of Alsidium corallinum C. Ag. in a Tyrrhenian Eutrophic Lagoon Dominated by Opportunistic Macroalgae, Marine Pollution Bulettin, 2012 Dec; 64(12): 2699-2707
Molinier R. and Picard J., 1952, Recherches sur les Herbiers de Phanerogames Marines du Littoral Méditerranéen Francais. Annales de l'Institut Oceanographique, XVII (3)
Piazzi L. and Balata D., 2008, The Spread of Caulerpa racamosa var. cylindracea in the Mediterranean Sea: An example of How Biological Invasion Can Influence Beta Diversity, Marine Environmental Research, 65(1): 50-61
Piazzi L., Balata D., Foresi L., Cristaudo C., Cinelli F. 2007. Sediment as a constituent of Mediterranean benthic communities dominated by Caulerpa racemosa var. cylindracea. Scientia Marina 71: 129-135
http://dx.doi.org/10.3989/scimar.2007.71n1129
Piazzi L., Balestri E. and Cinelli F., 1994, Presence of Caulerpa racemosa in the North-Western Mediterranean, Cryptogamie Algologie, 15(3): 183-189
Piazzi L. and Ceccherelli G., 2006, Persistence of Biological Invasion Effects: Recovery of Macroalgal Assemblages After Removal of Caulerpa racemosa var. cylindracea, Estuarine Coastal and Shelf Science, 68: 455-461
Piazzi L., Meinesz A., Verlaque M., Akçali B., Antolić B., Argyrou M, Balata D., Ballesteros E., Calvo S., Cinelli F., Cirik S., Cossu A., D'Archino R., Djellouli S.A., Javel F., Lanfranco E., Mifsud C., Pala D., Panayotidis P., Peirano A., Pergent G., Petrocelli A., Ruitton S., Žuljević A. and Ceccherelli G., 2005, Invasion of Caulerpa racemosa var. cylindracea (Caulerpales, Chlorophyta) in the Mediterranean Sea: an Assessment of the Early Stages of Spread, Cryptogamie Algologie, 26: 189-202
Ruitton S., Javel F., Culioli J.M., Meinesz A., Pergent G. and Verlaque M., 2005a, First Assessment of the Caulerpa racemosa (Caulerpales, Chlorophyta) Invasion Along the French Mediterranean Coast, Marine Pollution Bulletin, 50: 1061-1068
Sfriso A., Curiel D., 2007, Check-list of Seaweeds Recorded in the Last 20 Years in Venice Lagoon, and a Comparison with the Previous Records, Botanica marina, 50: 22-58
Tsiamisis K., Panayotidis P., Salomidi M., Pavlidou A., Kleinteich J., Balanika K., Kepper F.C., 2013, Macroalgal Community Response to Re-oligotrophication in Saronikos Gulf, Marine Ecology Progress Series, 472: 73-85
Verlaque M., 2001, Checklist of the Macroalgae of Thau Lagoon (Hérault, France), a Hot Spot of Marine Species Introduction in Europe, Oceanologica Acta, 24(1): 29-49
Verlaque M., Durand C., Huisman J.M., Boudouresque C.F. and Le Parco Y., 2003, On the Identity and Origin of the Mediterranean Invasive Caulerpa racemosa (Caulerpales, Chlorophyta), European Journal of Phycology, 38: 325-339
http://dx.doi.org/10.1080/09670260310001612592

 Author
Author  Correspondence author
Correspondence author
.png)

.png)


